Abstract
Background: Anticoagulation is the major therapeutic strategy in the management of patients with atrial fibrillation (AF). Thrombocytopenia is a frequent coexisting condition that may predispose to bleeding in these patients. However, the risk of bleeding in patients with AF and thrombocytopenia has not been well studied. Moderate thrombocytopenia (<100,000/μL) was an exclusion criteria in the clinical trials used to approve non-vitamin K oral anticoagulants for stroke prophylaxis. Similarly, studies examining warfarin's safety and efficacy in AF have not included significant numbers of thrombocytopenic patients. This paucity of data describing the safety of oral anticoagulants in patients with AF and thrombocytopenia represents a major challenge in clinical practice. Present treatment guidelines do not include specific guidance for anticoagulation in patients with these coexisting conditions. Thus, the purpose of this study was to compare rates of bleeding among patients with and without thrombocytopenia who begin oral anticoagulation for AF.
Methods: A propensity matched cohort study was designed to compare rates of bleeding among patients with thrombocytopenia who began oral anticoagulation for AF between 2015-2020 at a quaternary care hospital in Boston, MA against patients with AF starting anticoagulation in the absence of thrombocytopenia. The thrombocytopenic cohort was defined as having at least two platelet counts on separate days <100,000/μL in the period spanning the 12 weeks preceding the initiation of anticoagulation to 6 weeks following the initiation of anticoagulation. Patients with only one platelet count <100,000/μL were excluded. Individuals from the thrombocytopenic cohort were matched with patients (1:3 ratio) from the non-thrombocytopenic cohort using propensity matching on the basis of age, sex, CHA2DS2-VASc score, and anticoagulant agent used. Bleeding outcomes were determined by blinded manual chart review and verified by a second reviewer; all bleeding events were classified as per the International Society on Thrombosis and Haemostasis. The primary endpoint of the study was the cumulative incidence of clinically relevant bleeding, a composite of major bleeding and clinically relevant non-major bleeding (CRNMB), within one year of the initiation of anticoagulation.
Results: The study population included 1,075 patients who started oral anticoagulation for AF (274 with thrombocytopenia, 801 controls). Overall, 38% of the cohort was female, with a median age of 72 years; 14.6% of the total cohort had cancer and 2.8% had liver disease at the time they began anticoagulation. The median CHA2DS2-VASc score was 3 (IQR 2-4). Warfarin (48.2%) and apixaban (39.6%) were the anticoagulants used in the majority of patients. Notably, the thrombocytopenic cohort included more patients with significant comorbidities, such as cancer (23.0% vs 11.7%), liver disease (4.7% vs. 2.1%), and kidney disease (12.8% vs. 7.5%). There were a total 219 bleeding events recorded, including 90 (41.1%) major bleeding events and 122 (55.7%) CRNMB events. (Table 1)
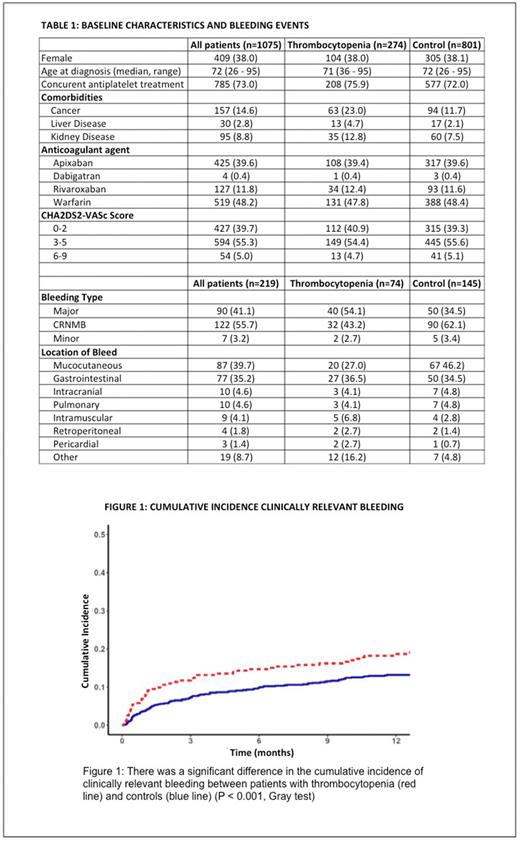
The cumulative incidence of clinically relevant bleeding at 1 year was significantly higher (18.3%; 95% CI, 13.6-22.9) in those with thrombocytopenia at the time of starting anticoagulation compared with the control cohort (13.2%; 95% CI, 10.9-15.6) (P < 0.001, Gray test). Similarly, the cumulative incidence of major bleeding at 1 year was 10.1% (95% CI, 6.5-13.7) in those with thrombocytopenia compared with 5.2% (95% CI, 3.7-6.8) among controls (P < 0.001, Gray test). (Figure 1)
Conclusions: Oral anticoagulation for AF in patients with thrombocytopenia at the time of starting anticoagulation significantly increased the risk of clinically relevant and major bleeding relative to patients with normal platelet counts. These findings support the need for further characterization of the risk-benefit profile of anticoagulation in this population to guide clinicians faced with this common clinical scenario.
Disclosures
Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Zwicker:Incyte: Research Funding; Sanofi: Consultancy; Quercegen: Research Funding; CSL: Consultancy; Parexel: Consultancy; Pfizer/BMS: Honoraria; Portola: Honoraria; Daiichi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal